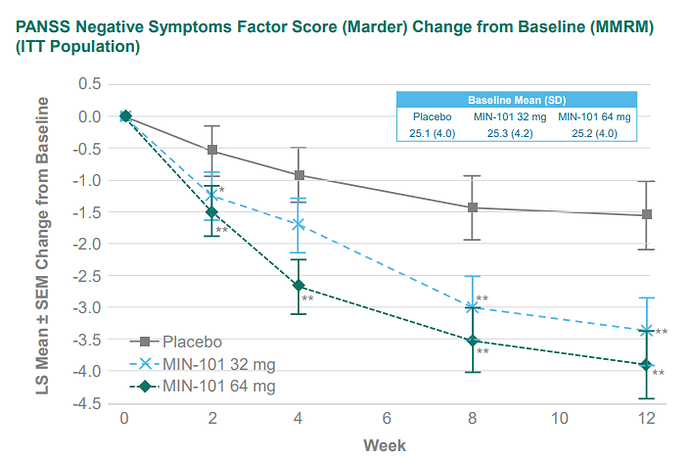
Published in The Journal of Clinical Psychiatry, study data suggest that MIN-101 may improve cognitive deficits in individuals with clinically significant negative symptoms of schizophrenia.
That’s good , thank you for posting @twinklestars , might be encouraging for some folks
who want more and better treatment options.
Thanks for the info @twinklestars!
@far_cry0 might like this
We really need some medication that is effective against negative and cognitive symptoms. . Holy jesus…!!
When iz this guys releasing. .
I’m pumped for this med!! It sounds so promising and like it could help a lot of people!! I am interested to try it as well but more because of its somewhat unique receptor binding profile.
It’s in phase 3. Probably sometime around 2020, 2021.
Okay lets see … it has to pass third 3rd phase too.
!!!
What will be the brand name of MIN-101?
They have named it Roluperidone.
From the risperidone family?
I don’t know how they decide on the names. They do often use the same prefix, roots or suffix in the naming of generics of a particular class (NSAIDs, beta blockers, antifungals, antibiotics, etc) Roluperidone is not going to be generic for quite awhile, I would guess. Maybe they are using the convention anyway.
iloperidone; paliperidone; risperidone - class atypical antipsychotic.
ITI 007’s name, Lumateperone also ends in -one, although they left off the id.
Nice name…!!!1515515151515
Hope min101 comes out being effective…!!!
I am rooting for it…!!! It has to pass 3rd phase with great improvement…!!! Good bless all of us…!!
The name of a generic is the name of the molecule.
Roluperidone is the name of a molecule. This is not a commercial name.
Hey @anon7255419 will this medicine pass its third phase…!!!
In that case, I don’t know what the brand name will be.
Thanks. Weird… it doesn’t show up with it’s molecular name on Google sites and/or on sites (except California) where in the US and Europe the phase III will be held. All I could find that the topline results of the phase III trials is expected mid 2019.